|
Introduction -Etomidate is an intravenous nonbarbiturate hypnotic -Developed in the 1970s, found to be an effective hypnotic [1] -Used as anesthesia induction agent and for first decade, used as sedative infusion -Discovered to cause adrenal suppression and increased mortality in some critically ill patients -Now predominately used as sedative medication in rapid sequence intubation (RSI) Structure - Available as 2mg/mL solution in 35% propylene glycol solution - Available in lipid solution in Europe, reportedly less pain on infusion - Sedative bolus at 0.2-0.4mg/kg, general anesthesia with continuous infusion of 30-100ug/kg/min Pharmacology/Pharmacokinetics [1] - pKa of 4.2 and is hydrophobic at physiologic pH - Formulated in 35% propylene glycol to increase solubility - 75% protein bound with large volume of distribution 74.9 L/kg due to high fat solubility - Three compartment model of pharmacokinetics for single bolus of etomidate 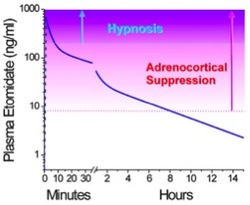 Compartment 1: rapid distribution into highly perfused tissue Compartment 2: redistribution into peripheral tissues (muscles) Compartment 3: terminal metabolism - Onset of action in <1min, duration is approximately 5 min - Metabolism dependent on hepatic esterase activity, which hydrolyzes to carboxylic acid and an ethanol leaving group - Carboxylate metabolite mostly excreted in urine - Metabolic t1/2 ranges from 2-5 hours - Elderly or ill patients require lower etomidate doses due to reduced protein binding and reduced clearance Mechanism of Action: - Non barbiturate hypnotic active at the GABAA receptor, specifically the β2 and β3 - Etomidate positively modulates GABAA activation by agonists - Slow postsynaptic current decay, prolonging postsynaptic inhibition - Blocks 11β-hydroxylase in the steroidogenesis pathway [2] - Nitrogen atom of the imidazole ring interacts with the active binding site of the enzyme Toxicity - Propylene glycol diluent is implicated in development of hyperosmolar metabolic acidosis when used in prolonged infusions - Cardiovascular side effects include hypotension and tachycardia, less significant than most other sedative agents - Both proconvulsant and anticonvulsant properties - Myoclonus- may be caused by etomidate interaction with glycine receptor at the spinal cord level - Adrenocortical suppression- etomidate blocks of 11β-hydroxylase leading to depressed adrenal production of cortisol and aldosterone
- Pain on infusion - Myoclonus - Postoperative nausea and vomiting (40%)- few studies have compared etomidate vs. other induction agents. One study has shown this to be comparable to barbituates and higher than propofol [1] - Adrenocortical inhibition- can manifest as hypotension refractory to fluids and vasopressors Management - Pain on infusion- consider pretreatment with 3mL 1% lidocaine - Nausea and vomiting- symptomatic treatment - Myoclonus- opioid (fentanyl derivatives, dezocine) [7] , magnesium [8] , ketamine [9] pretreatment - Adrenal suppression- should we treat with corticosteroids?
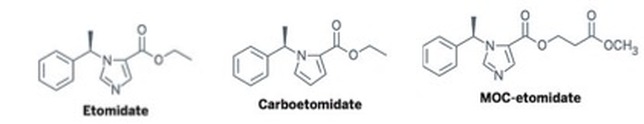
Future Directions Novel etomidate agents- Carboetomidate- contains pyrrole instead of imidazole, making binding 11β-hydroxylase less effective and thus reducing adrenal suppression [12] MOC-etomidate- Alteration made to ester moiety that is then rapidly metabolized, producing less profound and shorter-lasting adrenal suppression [12] Use in severe Cushing’s syndrome- several case studies outlining the use of etomidate infusions as low as 2.5mg/hr resulting in clinical improvement in hypercortisolism without causing sedative effects [13] References
1. Forman, S. Clinical and Molecular Pharmacology of Etomidate. Anesthesiology 2011; 114(3): 695-707. 2. de Jong FH, Mallios C, Jansen C, Scheck PA, Lamberts SW. Etomidate suppresses adrenocortical function by inhibition of 11 beta-hydroxylation. J Clin Endocrinol Metab 1984; 59: 1143–47. 3. Hildreth AN, Mejia VA, Maxwell RA, Smith PW, Dart BW, Barker DE. Adrenal suppression following a single dose of etomidate for rapid sequence induction: a prospective randomized study. J Trauma 2008; 65: 573–79. 4. Ledingham IM, Watt I. Influence of sedation on mortality in critically ill multiple trauma patients. Lancet 1983; 321: 1270. 5. P. Jabre, X. Combes, F. Lapostolle, M. Dhaouadi, A. Ricard-Hibon, B. Vivien, Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial, Lancet, Vol. 374, 2009, 293-300 6. Bruder EA, Ball IM, Ridi S, Pickett W, Hohl C. Single induction dose of etomidate versus other induction agents for endotracheal intubation in critically ill patients. Cochrane Database of Systematic Reviews 2015, Issue 1 7. Li, Z., He, L., Diny, Y., Chen, H. Dezocine pretreatment prevents myoclonus induced by etomidate: a randomized, double-blinded controlled trial, Anesthesiology 2015. 29(1):143-145. 8. Yelken, B., Un, B., Ceyhand, D. Prevention of etomidate-related myoclonus in anesthetic induction by pretreatment with magnesium. J. Res Med Sci 2011, 16(11):1490-1494. 9. Zhou, H., Wu, GN, Xu, HJ, Wu X. Low-dose Ketamine Pretreatment Reduces the Incidence and Severity of Myoclonus Induced by Etomidate: A randomized, Double-Blinded, Controlled Clinical Trial. Medicine 2016, 95(6) 10. Briegel, J. and CORTICUS study group. Hydrocortisone Therapy for Patients with Septic Shock. New Eng J Med 2008. 358(2): 111-124. 11. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012. Crit Care Med. 2013;41:580–637 12. Sney, J. Novel Etomidate Derivatives. Current Pharm Design 2012, 18: 6253-6256. 13. Price-Newell, J., Daniel, E. Therapy of Endocrine Disease: Steroidogenesis enzyme inhibitors in Cushing’s Syndrome. Eur J Endocrinol 2015. 172: 263-280. Other sources used include Goldfrank’s 10th edition, Haddad’s 3rd edition, and The Poison Review (www.thepoisonreview.com) Authored by: Dr. Cate Lounsbury, MD
1 Comment
|
Toxicology BlogAuthorEM Rotators on Toxicology Selected by Feedspot as one of the Top 20 Toxicology Blogs on the web
Archives
March 2018
Categories
All
Disclaimer: All images included on this blog are the sole property of CMC EM Residency and cannot be used or reproduced without written permission. Patient identifiers have been redacted/changed or patient consent has been obtained. Information contained in this blog is the opinion of the author and application of material contained in this blog is at the discretion of the practitioner to verify for accuracy.
|



 RSS Feed
RSS Feed
